The founders of GI Bionics (Drs. Gregersen and Kassab) have developed and patented several technologies that are now being developed towards clinical trials and FDA approval. Below is a short summary of three important patent-protected innovations.
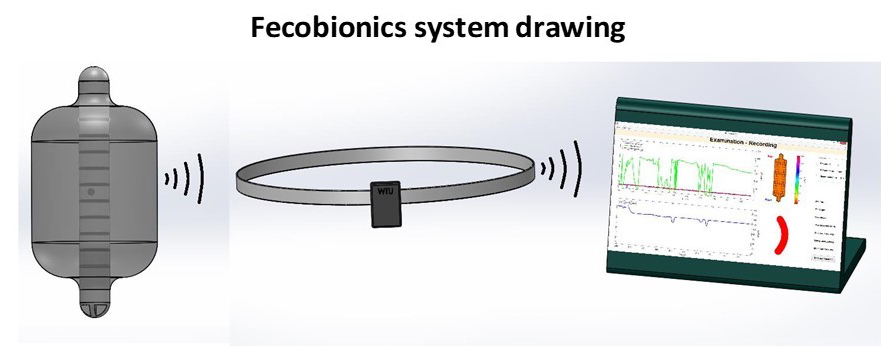
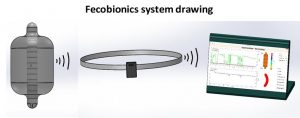
Fecobionics
Fecobionics constitutes a new paradigm for anorectal diagnostic testing, simulating normal defecation with a truly bionics device. Fecobionics is a simulated feces that measures pressures, anorectal angle and shape changes during defecation of the device. The technology integrates several current tests, provides an integrated framework for assessing defecation, and obviates the need for multiple tests of anorectal function. The impact is to use the safe, low cost, less invasive, low risk, radiation-free device to provide new understanding of defecation disorders to ultimately facilitate development of innovative biofeedback therapies that can be administered at home and monitored remotely. This can improve diagnostic and therapeutic modalities and reduce healthcare costs. Fecobionics is currently being tested in clinical trials and data has been obtained from more than 100 human subjects including patients with fecal incontinence and chronic constipation.
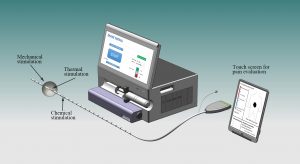
EndoXcite
EndoXcite is a technological development of the multimodal probe that has been tested in more than 600 human subjects including patients with functional chest pain, GERD and NERD. The multimodal probe stimulates the gastrointestinal tract mechanically, chemically and thermally and records pressures and dimensional changes. The new prototypes add new features such as gyroscopic measurement of the Hiss angle and high-resolution manometry. The impact is to use the new understanding of functional gastrointestinal disorders (FGIDs) to ultimately facilitate patient-specific treatments based on the extensive data obtained. This can improve diagnostic and therapeutic modalities and reduce healthcare costs.
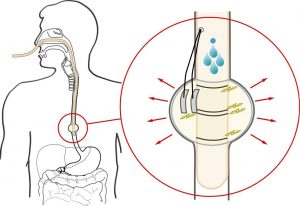
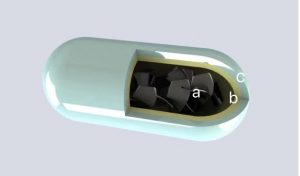
EndoXpand
Many treatment modalities exist for obesity spanning from diet to irreversible surgery. Intragastric balloons have been marketed but seem to lack efficacy long-term with modest weight loss. Furthermore, these bags must be inserted and removed endoscopically. It is our belief that the lack of long-term effect is due to accommodation to the constant volume in the stomach. We have developed an ingestible capsule that will expand in the stomach and dissolve within a specified period of time. By ingesting capsules in predefined schemes, the filling volume can easily be varied to avoid accommodation. Furthermore, electronics can be embedded in the capsule to monitor gastric physiology.